-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- About SMT
Main navigation
Unique Features
- Open cell design
- Flexible "S" Link
- Lipophilic everolimus drug
- Drug dose: 1.0 µg/mm2
- Optimum balance of hydrophilic and hydrophobic biodegradable polymers
- Precisely controlled, bi-phasic drug release profile
- Excellent coating integrity
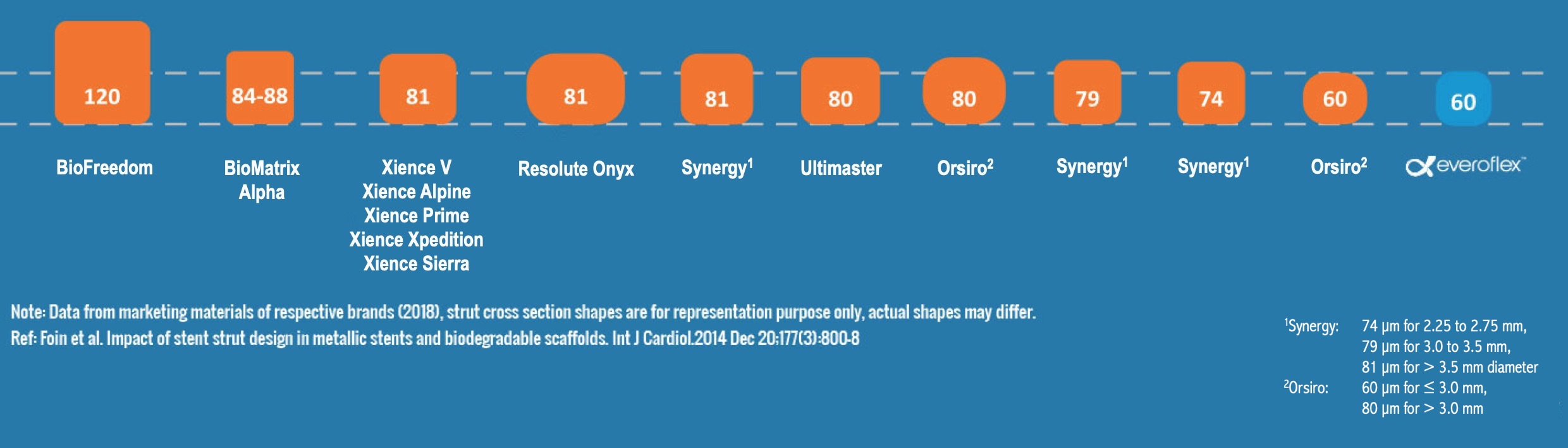
Lowest strut thickness across all the diameters

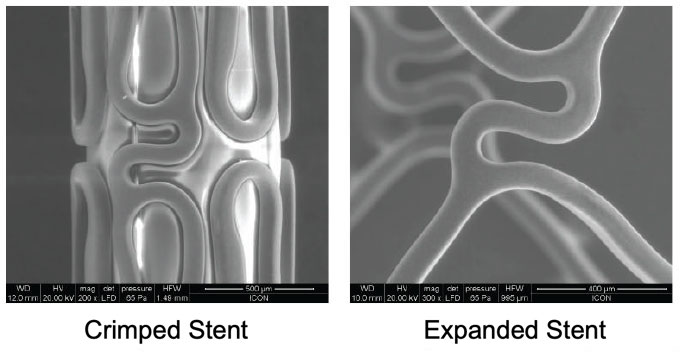
Coating Integrity
Process optimized to minimize webbing, bridging and other coating defects
Coating integrity maintained even after simulated use and post expansion up to RBP
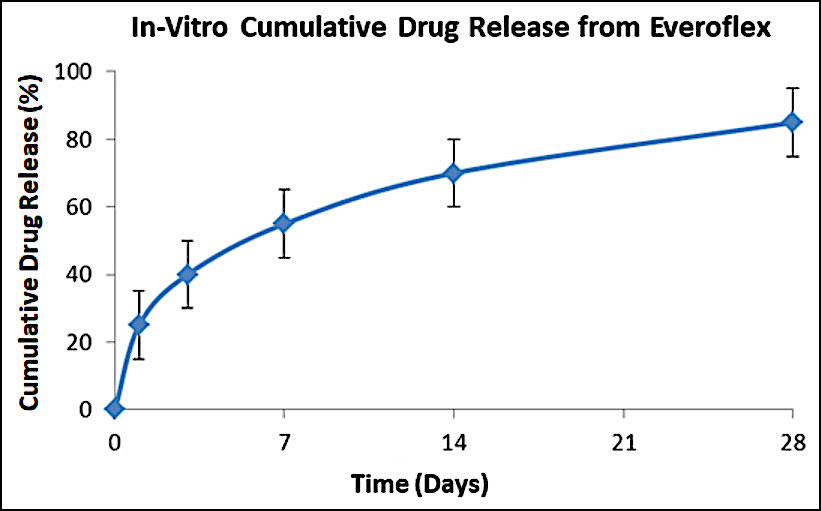
Drug release profile
- Drug: Everolimus
- Drug dose: 1.0 µg/mm2
- Release kinetics
- About 80% of drug is released at 4 weeks in biological media while 100% drug is released at a slow rate within 3 months.
- The initial moderate level of everolimus drug release from middle layer coating helps to inhibit early phase of neointimal hyperplasia.
- Controlled drug release kinetics from base layer coating is beneficial to maintain the effective amount of drug level in the arterial tissues which is required to prevent smooth muscle cell proliferation.
Available sizes
| Available stent lengths (mm) | 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 |
| Available stent diameter (mm) | 2.00, 2.25, 2.50, 2.75, 3.00, 3.50, 4.00, 4.50 |
Everoflex is a trademark of Sahajanand Medical Technologies Ltd. or its affiliates. Specifications are subject to modification, revision and improvement.
BioFreedom and BioMatrix Alpha are trademarks of Biosensors International. Xience V, Xience Alpine, Xience Prime, Xience Xpedition and Xience Sierra are trademarks of the Abbott Group of Companies. Resolute Onyx is a trademark of Medtronic, Inc. or it's affiliates. Synergy is a trademark of Boston Scientific Corporation or its affiliates. Ultimaster is a trademark of Terumo Corporation. Orsiro is a trademark of Biotronik SE.


